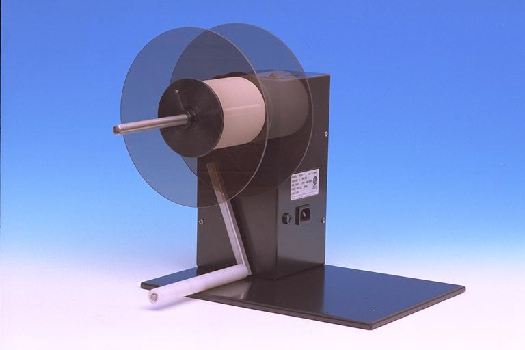
Electric Rewinder Models GLU-100 & Model GLR-100
- Auto-switching Universal Power Supply for immediate adaptability anywhere in the world
- Compatible with all Direct Thermal and Thermal Transfer printers
- Electronic Speed Control will automatically synchronise winding speed to the speed of your printer
- Provides passive winding without skewing print or barcodes on your labels
- Rewind up to 61cm per second
- Easily handles labels up to 23cm wide
- Wind labels face in or face out
SPECIFICATIONS
Speed – 2.5 to 61.5cm/sec
Roll Width – 2.5cm to 23cm
Core Size – 7.6cm
Roll Diameter – 35cm
Physical
Length – 42cm
Width – 33cm
Height – 28cm
Weight – 7kg
Electrical – 100 – 250 Volt 47 – 63Hz
Environment
Temperature – 5 to 40º
Adobe Reader is required to open and view PDF files. This can be downloaded free from adobe.com.
Available Documents
GLRGLU100-
-
- Applies label up to 6″ high x 12″ long
-
-
-
- Handles up to 45 products/minute
-
-
-
- Placement accuracy of 0.03″
-
- Depth – No Maximum
- Max. Reel Diameter – Max. 225mm
- Core Size – Min. 25mm Ideal 76mm
Bottle-Matic Label Applicators

Bottle-Matic bottle labelers enable you to label all kinds of products by simply inserting the container and depressing a foot-switch. Labels are applied at over 4.5” per second – most water bottles are labeled in less than two seconds. The operator then removes the bottle and inserts another to start the procedure again. These automated labeling systems make it easy to label wine bottles, craft beer bottles, water bottles and most other round products.
View ProductWeber 114 One-Sided/Two-Sided Labelling System

The brand-new Weber 114 system provides a simple, cost-effective solution to low volume labelling for applying labels to one or two sides of a straight-walled product or package. The Model 114 can be added to in-place production lines or operated manually as a stand-alone applicator.
The Weber 114 is easy to use and can be set-up quickly. During application, labels are peeled away from the liner and securely attached to products using the wipe-on label applicators.
The unit boasts robust steel construction making it perfect for lower-volume, intermittent labelling projects or multiple-shift larger-scale operations. This is a very reliable wipe-on application system that is built to last.
View Product
Legi-Air 5300 Tyre Label Printer-Applicator

According to an EU regulation all new tires produced in or after January 2012 and placed on the market within the EU must bear a standardized EU sticker, such as those already seen on washing machines and refrigerators. The new label must contain precise details with seven grades (A-G) of fuel efficiency, wet grip, and exterior noise when in motion (in dB), respectively.
View ProductElectric Semi-Automatic Label Dispensers

These machines can dispense up to a maximum web width of 152mm butt cut or die cut and adjustments for different size labels are made easily
and without the need for tools.
View Product